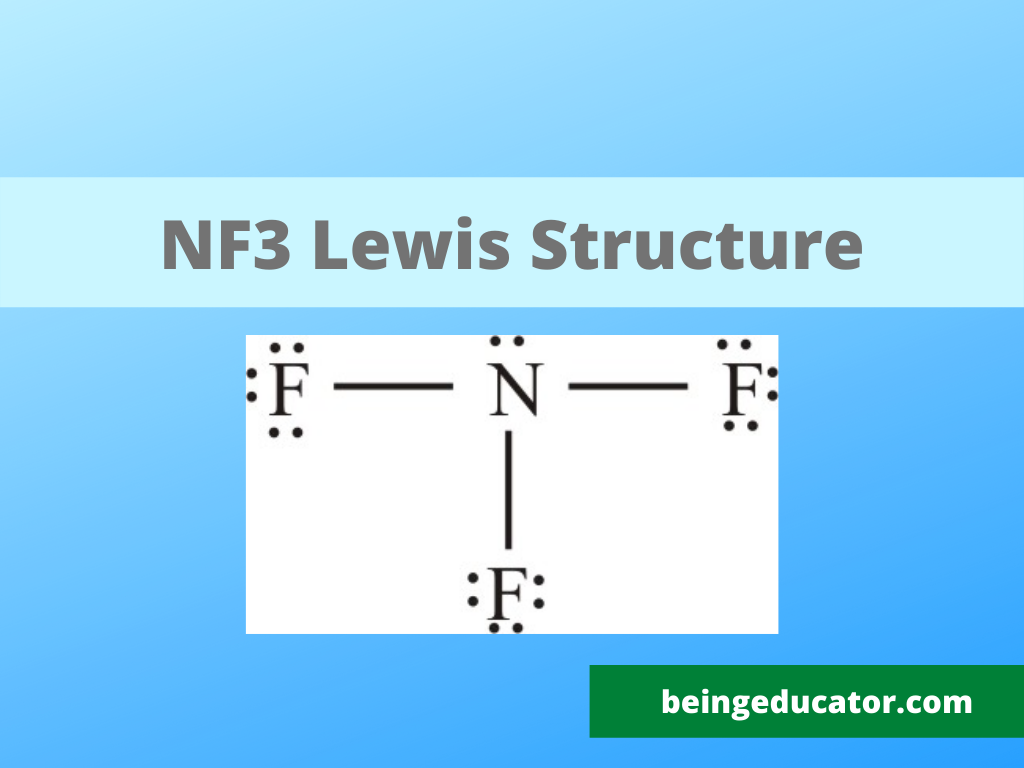
Lewsis Structure of Nitrogen Triflouride
Nitrogen trifluoride is chemically formed by three fluorine atoms and a single nitrogen atom. The nf3 lewis structure explains three N-F bond pairs and a single lone pair of electrons on the nitrogen atom. The lewis structure is peculiar because both the atoms in the NF3 are electronegative (fluorine more than nitrogen) and are chemically bonded to form a stable molecule. The exciting thing about the nf3 molecule is that the nitrogen has five while the fluorine has one valence electron. Hence, both atoms satisfy the octet rule by bonding three fluorine with a single nitrogen atom.
The importance of nitrogen came into account due to the reason because it can create the greenhouse effect and can help in raising the temperature of the greenhouse. If you are about to handle the NF3 gas, you must follow preventive measures because this gas is highly toxic and causes serious health concerns when inhaled.
The lewis structure of nitrogen trifluoride gives a graphical glimpse of the types of atoms involved in chemical bonding and also explains the molecular geometry of the nf3 molecule. A stable nf3 molecule is formed when three fluorine molecules bond with a single nitrogen atom. The particular step in determining the lewis structure is to find out the number of valence electrons in any molecule. Valence electrons are referred to as the electrons present in an atom’s last shell(Outermost). If you are interested to learn about the polarity click on the HF Polar or Non Polar for details
Steps of Determining the Lewis Structure of NF3
We are about to find out the lewis dot structure of nf3 and find out the molecular shape and polarity of NF3. NF3 is a halide of nitrogen gas with the nitrogen belonging to Group 15 and Fluorine belonging to group 17 of the modern periodic table. The nitrogen molecule needs three electrons, while fluorine requires only one electron to complete its octet.
The first rule to draw the lewis structure is to determine the total valence electrons. In this case, nitrogen has five, and fluorine has 7 with total valence electrons 5+7×3= 5+21=26.
The second step is to find out the central atom to place in the lewis structure. In this case, seeing the stability and electropositivitty nitrogen comes in the centre o the nf3 molecule. The other reason that nitrogen is the central atom is that it correctly showed the bonding electrons in the true sense, and otherwise, it doesn’t seem correct.
The next step is to find out the number of lone pairs and bond pairs in the nf3 molecule.
Now mark any charge on the atom if present and try to finalise the lewis structure. The assigning of charge also helps in determining the polarity of any molecule. The Net charge on NF3 is zero, so we can say that it is a neutral compound
The final step is to find out the stability of any molecule, and if the molecule is stable, then the lewis structure is correctly explained.
How to find the total valence electron pair in the NF3 molecule?
The formula to calculate the total valence electron pair in NF3 is to divide the number of valence electrons to two, and in the case of NF3, the valence electron pairs in NF3 are thirteen.
Molecular Geometry
The lewis structure of NF3 tells us about the shape and molecular geometry. According to the VESPER theory, the shape of NF3 is trigonal bipyramidal, with Nitrogen being the central atom and the three fluorine molecules surrounding it.
Polarity of NF3
The lewis diagram shows that the NF3 molecule shows polar nature. The other thing is the difference in electronegativity also tells us about the polar nature of the nf3 molecule.
Leave a Reply