Oxygen Lewis Dot Structure
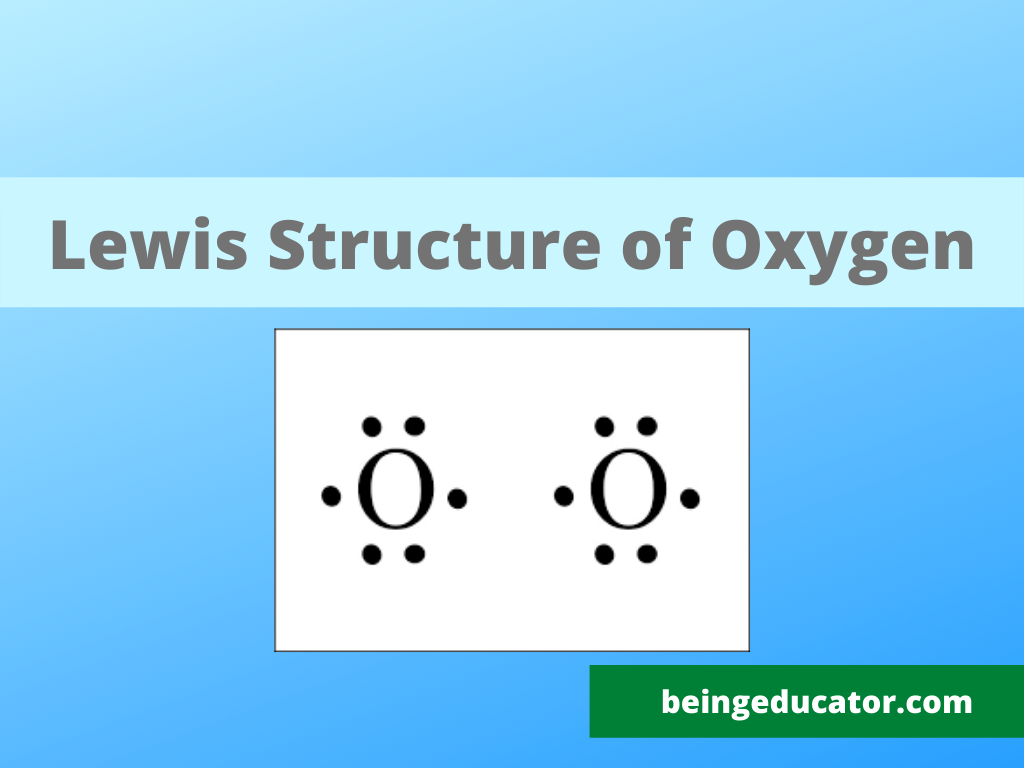
The oxygen molecule’s lewis structure is quickly drawn if you know the molecular structure and number of valence electrons in the oxygen element. The oxygen element is one of the life elements because cellular respiration occurs only in oxygen molecules. Oxygen is found mainly in the gaseous state and is called life gas because more than 80% of the living creatures require it to live.
How many valence electrons are there in the oxygen molecule?
The monoatomic form is oxygen is highly unstable, and on most occasions, two atoms of oxygen bonded to become a stable oxygen molecule. The single oxygen atom has six valence electrons, and then the oxygen molecule has 12 valence elections with four non-bonded electron pairs.
Geometric Shape of Oxygen atoms
The oxygen molecule is bonded when two valence electrons are shared by both atoms, resulting in a double covalent bond. The geometrical shape of the oxygen atom is linear, with both atoms stably bonded to attain the electronic configuration of the nearest noble gas.
Hybridization of Oxygen Molecule
You are wondering about the hybridization of oxygen molecules, and it shows sp2 hybridization with one s and two p orbitals mixing to give a relatively stable sp2 hybridization. The importance of hybridization in determining lewis structure and geometry of molecules could answer many complex chemistry questions.
Polarity of oxygen molecule
The oxygen atom is non-polar because there is no significant electronegativity difference between both oxygen atoms. Hence, no dipole is created when someone asks you whether oxygen is polar or non-polar; you straightforward answer that the oxygen molecule is non-polar. When oxygen bonds with other low electronegative elements, it may form polar molecules due to the significant electronegativity difference.
You can learn about the polar nature of PCl3 a polyatomic molecule with the property to be flammable and use preparation of various chemicals
Steps of Drawing the lewis structure of Oxgen Molecule
The first step in drawing the lewis structure of any molecule is determining the number of a molecule. In the case of oxygen is a diatomic molecule and comprises two atoms. The comprised second step is to find out the number of electrons in the outermost shell of the oxygen atom. Thus the oxygen atom has six valence electrons, and the oxygen molecule has 12 valence electrons.
The third step is to calculate the bonding and lone pair electrons
Now the electron bonding is estimated, it further proceeds with the marking of centre atoms with the two molecules of oxygen atoms involved with no centre atom, and thus, both are bonded equidistant with each other
The final proceedings to drawing the lewis structure of oxygen mark any charge on the bonding atoms. The two oxygen atoms with no charge, so the oxygen molecule is electrically neutral.
The last step is determining the molecule’s stability, and oxygen gas is a highly stable molecule, and that’s why it is found on earth atmosphere in such a large percentage.
Which type of bond is present in oxygen atoms?
Oxygen belongs to group 16 elements, mostly called the chalcogens, with the two covalent bonds present between both oxygen atoms.-
Summary
Oxygen is required for the continuity of life on Earth due to the large species requiring cellular respiration. Oxygen is pure elemental form is relatively unstable and in majority found in elemental form (oxygen and Ozone) with 12 valence electrons. The lewis structure of oxygen tells us that it is non-polar moleculemolecules2 hybridization, and molecular geometry is a linear molecule.
Leave a Reply