Is Ammonia a Polar?
Ammonia symbolically represented as NH3 is colourless gas widely used in many commercial activities like manufacturing nitrogenous fertilizers, as a cleaning agent, and producing a variety of nitrogenous chemicals. Most of the students are confused when they are asked if ammonia is polar or nonpolar. In chemistry, the term polarity means the existence of dipoles within the molecule. Dipoles mean that there is an unequal distribution of electrons shared among different atoms in a molecule. The existence of dipoles in a compound means that it is polar. In ammonia there are three dipoles and due to which ammonia molecule is a polar compound.
Why NH3 is a Polar Compound?
The polar nature of ammonia marks many questions among students but the major reason for the polarity of the ammonia molecules is the difference in electronegativity of the bonding atoms.
Is nh3 polar?
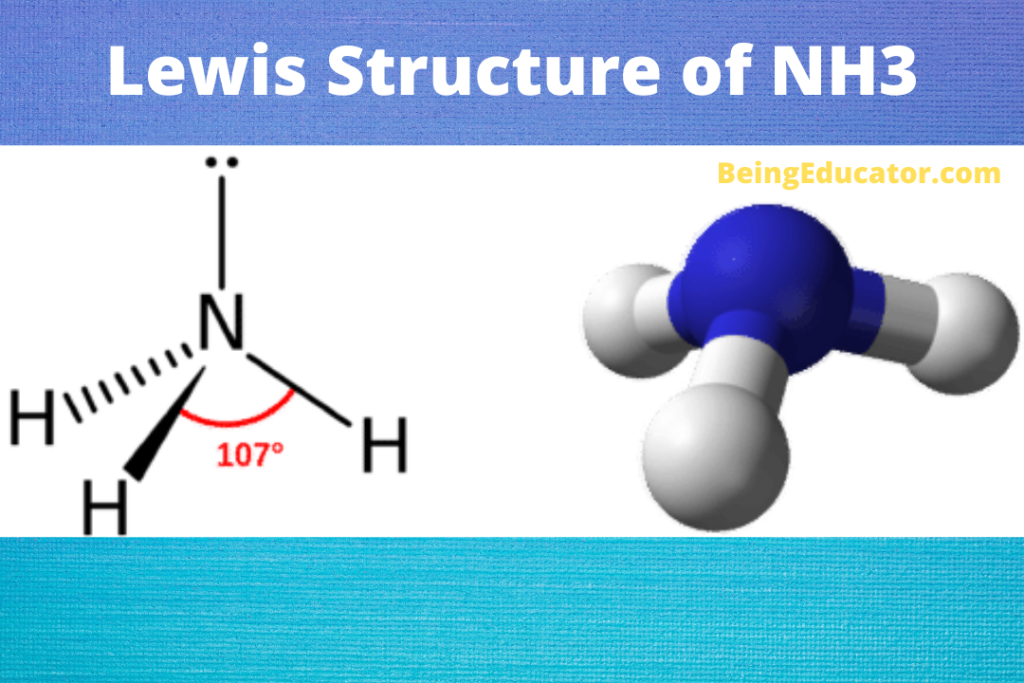
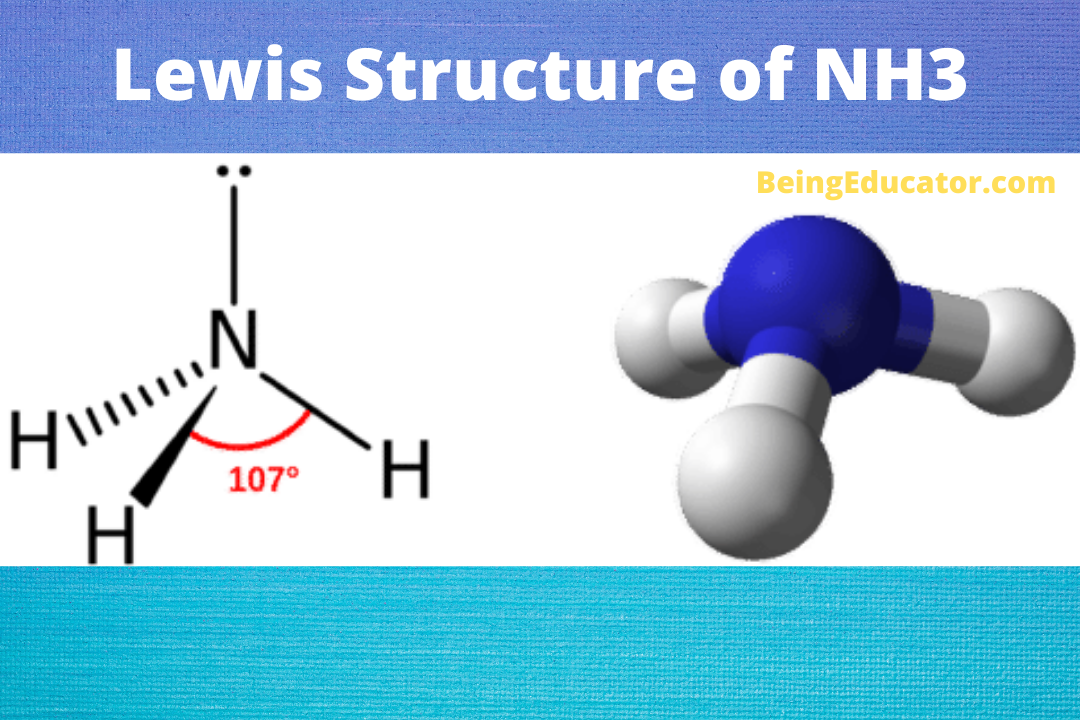
Yes, Ammonia is a polar molecule. The polarity of nh3 is mostly depicted by the electron density difference between nitrogen and hydrogen atoms. The three N-H bonds each having separate dipoles makes ammonia a polar compound. The lewis structure of ammonia perfectly explains the polarity of nh3 as the three are a total of 8 valence electrons (5 from nitrogen and 3 from hydrogen). The lone pair of electrons (Nitrogen atom) makes the shape of ammonia as trigonal pyramidal. The existence of three dipoles in ammonia molecules makes it a strongly polar molecule. If you want to check the molecular structure and geometry of krf2 visit KrF2 Polar or Nonpolar
Ammonia is also present in living bodies of living organisms because it is one of the by-products of amino acid metabolism. As a result of protein metabolism ammonia is produced but it is very toxic due to which it is removed as a nitrogenous waste from the bodies of living organisms. The organisms that produce ammonia as a nitrogenous waste material are called ammonotelic.
Ammonia has a lot of commercial applications and it is manufactured by Famous Harbers Process. The Hydrogen and Nitrogen Gases under high pressure and temperature and in the presence of catalysts are used to manufacture ammonia gas. This reaction was the invention of German chemist Fritz Harber and was given a Nobel prize in Chemistry for ammonia gas manufacturing.
The polar and nonpolar nature can only be explained by the lewis structure and the difference in electronegativity among the bonding atoms. In water molecules, oxygen has more electronegativity and hydrogen has less electronegativity due to which the two dipoles are created with oxygen-carrying more negative charges and hydrogen with a more positive charge. The distribution of charges is only due to the unequal sharing of electrons.
Ammonia is a polar molecule and its polarity is established due to its molecular structure and difference in electronegativity between nitrogen and hydrogen. Nitrogen with higher electronegativity(3.04) tends to gather more electronic clouds and thus creates a partial negative charge on it and hydrogen being less electronegative(2.20) gets a partial positive charge on it.
The Lewis structure of Ammonia shows three Nitrogen and Hydrogen bonds covalently linked by single covalent bonds and a lone pair of electrons. The shape of ammonia molecule as per lewis structure is trigonal pyramidal and bond angle of 107.8
You can check the CS2 Lewis structure by simply clicking on the blog post specifically curated to clear ambiguity related to the carbon disulfide lewis structure.
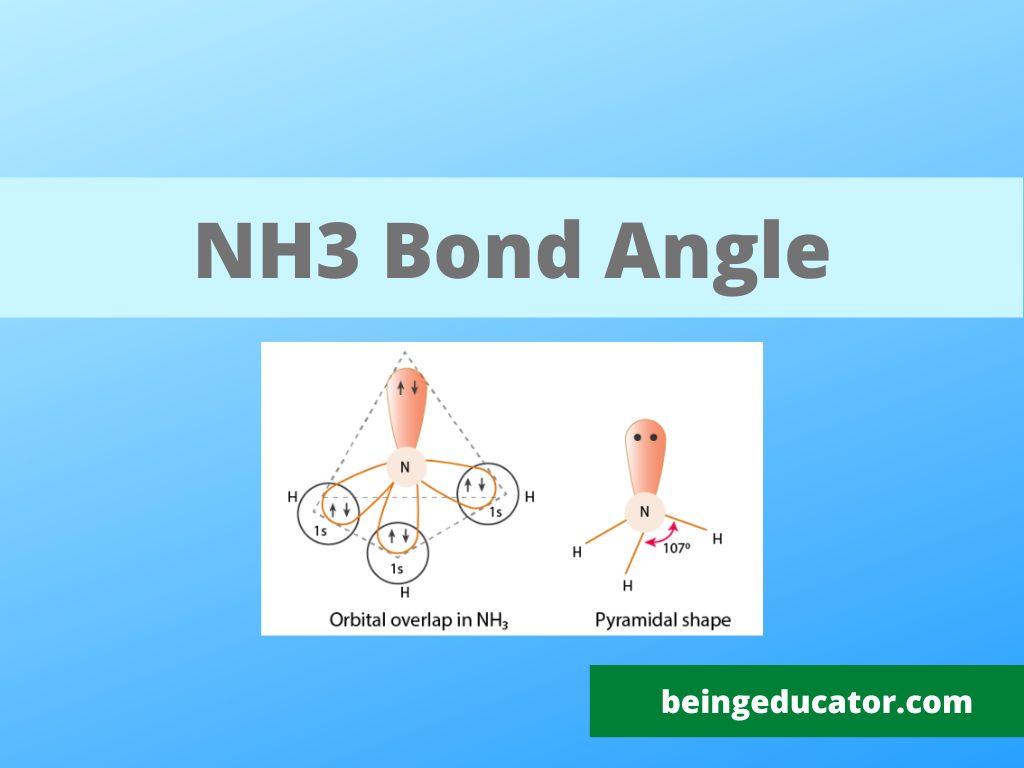
Bond Angle of NH3
The bond angle of ammonia can easily be explained by learning the hybridization. Hydrogen belongs to group 1 element thus it can complete the octet by easily gaining one electron. On the other hand, nitrogen belongs to the halogen family group 15A and has seven valence electrons and needs one to complete the octet. The octet is completed when one s and three p orbital are combined to form sp3 hybridization.
Difference between polar and nonpolar molecules
The polar molecules mostly are created due to the unequal electron sharing between the bonding atoms. A lot of students confuse polar and nonpolar compounds with ionic and covalent compounds.
Ionic bonds are formed between the two atoms when a complete electron transfer occurs, thus creating two dipoles. The atoms in ionic compounds usually have unequal electronic charge distribution or have appreciable electronegativity differences. The difference in electronegativity thus creates two dipoles with one being a partial negative charge on the other a partial positive charge and ionic compounds are formed.
On the contrary, the covalent bond is formed due to mutual electron sharing between the bonding atoms. The covalent bond may be polar or nonpolar and it all depends on the electronegativity. The covalent bond is said to be polar if the difference in electronegativity between the bonding atoms creates two oppositely charged dipoles having partial positive and negative charges and if the bonding atoms have the same electronegativity the compound is said to be polar. We can summarize that polarity of a molecule is dependent on the difference in electronegativity between the atoms in a molecule.
How do you know NH3 is polar?
The electronegativity difference between the nitrogen and hydron atoms creates multiple dipoles and gives ammonia a polar nature.
Conclusions
Ammonia (NH3) is a polar molecule and it shows polar nature due to the difference in electronegativity between nitrogen and hydrogen. The Three Hydrogen and nitrogen dipoles make ammonia a polar molecule.




8 thoughts on “Is NH3 Polar or Non Polar (Simply Explained)”