L’ammoniaca, con la formula chimica NH3, è una delle sostanze chimiche più comunemente conosciute e utilizzate. Ma la domanda che molti si pongono è: l’ammoniaca è un acido o una base? In questo articolo, esploreremo in dettaglio le proprietà chimiche dell’ammoniaca, la sua natura acida o basica, e le implicazioni pratiche e teoriche di questa caratteristica.
Cos’è l’Ammoniaca?
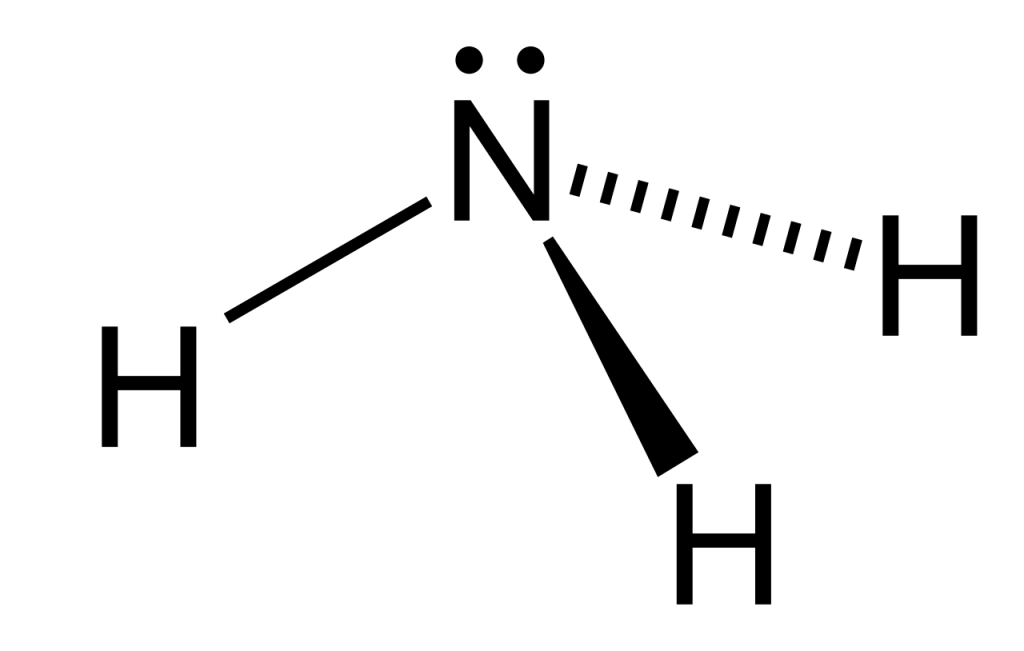
L’ammoniaca è un composto chimico costituito da un atomo di azoto (N) legato a tre atomi di idrogeno (H). La sua formula chimica è NH3. Si presenta come un gas incolore con un odore pungente e caratteristico. L’ammoniaca è altamente solubile in acqua, formando una soluzione che è comunemente conosciuta come idrossido di ammonio (NH4OH).
La Teoria degli Acidi e delle Basi
Per comprendere se l’ammoniaca è un acido o una base, è essenziale avere una comprensione di base delle teorie degli acidi e delle basi. Le principali teorie sono tre:
- Teoria di Arrhenius: Secondo questa teoria, un acido è una sostanza che rilascia ioni H+ (ioni idrogeno) in soluzione acquosa, mentre una base è una sostanza che rilascia ioni OH- (ioni idrossido).
- Teoria di Brønsted-Lowry: Questa teoria amplia il concetto di acidi e basi definendo un acido come un donatore di protoni (H+) e una base come un accettore di protoni.
- Teoria di Lewis: Secondo questa teoria, un acido è una sostanza che può accettare una coppia di elettroni, mentre una base è una sostanza che può donare una coppia di elettroni.
NH3 come Base
Secondo le teorie di Brønsted-Lowry e Lewis, l’ammoniaca è considerata una base. Ecco perché:
Teoria di Brønsted-Lowry
Secondo la teoria di Brønsted-Lowry, una base è una sostanza che accetta protoni. Quando l’ammoniaca viene disciolta in acqua, reagisce con le molecole di acqua accettando un protone (H+) da una molecola d’acqua. Questo porta alla formazione dello ione ammonio (NH4+) e dello ione idrossido (OH-):
NH3+H2O→NH4++OH−\text{NH3} + \text{H2O} \rightarrow \text{NH4}^+ + \text{OH}^-NH3+H2O→NH4++OH−
Questa reazione dimostra chiaramente il comportamento basico dell’ammoniaca, poiché accetta un protone dalla molecola d’acqua.
Teoria di Lewis
Secondo la teoria di Lewis, una base è una sostanza che può donare una coppia di elettroni. L’atomo di azoto in NH3 ha una coppia di elettroni solitaria, che può essere donata per formare un legame con un’altra sostanza. Ad esempio, l’ammoniaca può reagire con un acido di Lewis come il trifluoruro di boro (BF3):
NH3+BF3→H3N-BF3\text{NH3} + \text{BF3} \rightarrow \text{H3N-BF3}NH3+BF3→H3N-BF3
In questa reazione, l’ammoniaca dona la sua coppia di elettroni solitaria all’acido di Lewis BF3, dimostrando ancora una volta il suo comportamento basico.
NH3 come Acido
Sebbene l’ammoniaca sia prevalentemente conosciuta come una base, ci sono situazioni in cui può agire come un acido. Secondo la teoria di Brønsted-Lowry, un acido è una sostanza che può donare un protone. L’ammoniaca può agire come un acido molto debole in alcune reazioni.
Ad esempio, l’ammoniaca può donare un protone a basi molto forti, come l’anione ammidico (NH2^-):
NH3+NH2−→NH2−+NH3\text{NH3} + \text{NH2}^- \rightarrow \text{NH2}^- + \text{NH3}NH3+NH2−→NH2−+NH3
In questa reazione, l’ammoniaca dona un protone all’anione ammidico, comportandosi come un acido molto debole.
L’Equilibrio Chimico e il pH delle Soluzioni di NH3
Quando l’ammoniaca è disciolta in acqua, forma un equilibrio chimico tra NH3, NH4+, e OH-. La costante di equilibrio per questa reazione è chiamata costante di dissociazione basica (Kb):
NH3+H2O⇌NH4++OH−\text{NH3} + \text{H2O} \rightleftharpoons \text{NH4}^+ + \text{OH}^-NH3+H2O⇌NH4++OH−
La Kb dell’ammoniaca è 1.8 x 10^-5, indicando che l’ammoniaca è una base debole. Il pH di una soluzione acquosa di ammoniaca è tipicamente basico, variando tra 11 e 12 a seconda della concentrazione.
Applicazioni dell’Ammoniaca
Le proprietà basiche dell’ammoniaca la rendono utile in molte applicazioni industriali e domestiche:
- Produzione di Fertilizzanti: L’ammoniaca è un componente chiave nella produzione di fertilizzanti, come il nitrato di ammonio.
- Pulizia: L’ammoniaca è spesso utilizzata nei prodotti per la pulizia domestica a causa della sua capacità di neutralizzare gli acidi e dissolvere i grassi.
- Trattamento delle Acque: L’ammoniaca è utilizzata per regolare il pH delle acque e per la rimozione di inquinanti come i nitrati.
- Produzione di Plastica: L’ammoniaca è un precursore nella sintesi di numerose sostanze chimiche utilizzate nella produzione di materie plastiche.
Sicurezza e Maneggiamento
L’ammoniaca, nonostante sia utile, può essere pericolosa se non maneggiata correttamente. È importante seguire le linee guida di sicurezza quando si lavora con l’ammoniaca:
- Ventilazione Adeguata: Utilizzare l’ammoniaca in aree ben ventilate per evitare l’accumulo di gas.
- Dispositivi di Protezione Individuale: Indossare guanti, occhiali protettivi e, se necessario, maschere per evitare il contatto diretto con l’ammoniaca.
- Stoccaggio Sicuro: Conservare l’ammoniaca in contenitori sigillati e resistenti, lontano da fonti di calore e fiamme.
- Pronto Soccorso: In caso di esposizione accidentale, è importante sciacquare immediatamente la zona interessata con abbondante acqua e consultare un medico.
Conclusioni
L’ammoniaca (NH3) è prevalentemente una base secondo le teorie di Brønsted-Lowry e Lewis. Tuttavia, in condizioni particolari, può comportarsi come un acido molto debole. La sua natura basica è confermata dalla sua capacità di accettare protoni e donare coppie di elettroni. Le applicazioni pratiche dell’ammoniaca sono molteplici, rendendola una sostanza chimica di grande importanza industriale e domestica. Tuttavia, è essenziale maneggiare l’ammoniaca con cautela per evitare rischi per la salute.
Parole Chiave
- NH3
- Ammoniaca
- Acido o base
- Teoria di Brønsted-Lowry
- Teoria di Lewis
- Proprietà chimiche dell’ammoniaca
- Applicazioni dell’ammoniaca
- Sicurezza ammoniaca
Questo articolo mira a fornire una comprensione completa delle proprietà chimiche dell’ammoniaca, aiutando i lettori a capire meglio se NH3 è un acido o una base. Con parole chiave ben scelte e un contenuto chiaro e dettagliato, speriamo di migliorare la vostra conoscenza su questo argomento chimico fondamentale.
4o
Can you write a 2000 words detailed blog post on topic “nh3 base forte o debole” in Italian language. Make the article seo friendly and easy to understandChatGPT
NH3: Base Forte o Debole? Un’Analisi Dettagliata
Introduzione
L’ammoniaca, nota chimicamente come NH3, è un composto largamente utilizzato sia in ambito industriale che domestico. Una delle domande più frequenti tra chi si avvicina allo studio della chimica è se l’ammoniaca sia una base forte o debole. Questo articolo ha l’obiettivo di fornire una risposta dettagliata e comprensibile a questa domanda, esplorando le proprietà chimiche dell’ammoniaca, il suo comportamento in soluzioni acquose e il suo ruolo in diverse applicazioni.
Che Cos’è una Base?
Per comprendere se l’ammoniaca è una base forte o debole, è importante chiarire cosa si intende per base in chimica. Esistono diverse teorie che definiscono le basi:
- Teoria di Arrhenius: Una base è una sostanza che rilascia ioni OH- (ioni idrossido) in soluzione acquosa.
- Teoria di Brønsted-Lowry: Una base è una sostanza che accetta protoni (H+).
- Teoria di Lewis: Una base è una sostanza che dona una coppia di elettroni.
NH3: Struttura e Proprietà
L’ammoniaca è un composto chimico formato da un atomo di azoto legato a tre atomi di idrogeno. La sua formula chimica è NH3. È un gas incolore con un odore pungente caratteristico e altamente solubile in acqua, formando una soluzione chiamata idrossido di ammonio (NH4OH).
La presenza di una coppia di elettroni non condivisa sull’atomo di azoto permette all’ammoniaca di comportarsi come una base secondo le teorie di Brønsted-Lowry e Lewis.
NH3: Base Forte o Debole?
Teoria di Brønsted-Lowry
Secondo la teoria di Brønsted-Lowry, una base è una sostanza che accetta protoni. Quando l’ammoniaca è disciolta in acqua, reagisce con le molecole d’acqua accettando un protone (H+) da una molecola d’acqua. Questa reazione forma ioni ammonio (NH4+) e ioni idrossido (OH-):
NH3+H2O→NH4++OH−\text{NH3} + \text{H2O} \rightarrow \text{NH4}^+ + \text{OH}^-NH3+H2O→NH4++OH−
Questa reazione dimostra il comportamento basico dell’ammoniaca. Tuttavia, per determinare se l’ammoniaca è una base forte o debole, dobbiamo considerare la sua costante di dissociazione basica (Kb).
Costante di Dissociazione Basica (Kb)
La Kb è una misura della forza di una base. Una Kb alta indica una base forte, mentre una Kb bassa indica una base debole. La Kb dell’ammoniaca è 1.8 x 10^-5, che è relativamente bassa. Questo valore indica che l’ammoniaca è una base debole. Una base forte, come l’idrossido di sodio (NaOH), ha una Kb molto alta, mentre l’ammoniaca si dissocia solo parzialmente in soluzione acquosa, confermando la sua natura di base debole.
pH delle Soluzioni di NH3
Il pH di una soluzione è una misura della sua acidità o basicità. Le soluzioni di basi forti hanno un pH molto alto (superiore a 11-12), mentre le basi deboli, come l’ammoniaca, hanno un pH compreso tra 9 e 11. Il pH di una soluzione di ammoniaca dipende dalla sua concentrazione, ma tipicamente è intorno a 11 per soluzioni concentrate. Questo pH moderatamente alto è coerente con il comportamento di una base debole.
Confronto tra NH3 e Basi Forti
Per meglio comprendere la differenza tra una base forte e una base debole, confrontiamo l’ammoniaca con una base forte comune, come l’idrossido di sodio (NaOH).
Dissociazione in Soluzione
- NH3: Come già menzionato, l’ammoniaca si dissocia parzialmente in soluzione acquosa: NH3+H2O→NH4++OH−\text{NH3} + \text{H2O} \rightarrow \text{NH4}^+ + \text{OH}^-NH3+H2O→NH4++OH− Solo una piccola frazione delle molecole di ammoniaca accetta protoni, il che rende la soluzione meno basica rispetto a quella di una base forte.
- NaOH: L’idrossido di sodio si dissocia completamente in soluzione: NaOH→Na++OH−\text{NaOH} \rightarrow \text{Na}^+ + \text{OH}^-NaOH→Na++OH− Ogni molecola di NaOH rilascia ioni OH-, rendendo la soluzione fortemente basica.
pH delle Soluzioni
- NH3: Il pH delle soluzioni di ammoniaca è generalmente intorno a 11, indicando una basicità moderata.
- NaOH: Il pH delle soluzioni di idrossido di sodio può facilmente superare 13-14, indicando una forte basicità.
Applicazioni delle Basi
Le basi forti e deboli trovano applicazioni diverse a causa delle loro proprietà chimiche.
- Basi Forti (NaOH): Utilizzate principalmente in applicazioni industriali dove è necessaria una reazione altamente basica, come nella produzione di saponi, detergenti, e nella neutralizzazione degli acidi forti.
- Basi Deboli (NH3): Utilizzate in applicazioni dove una base moderata è più adatta. Ad esempio, l’ammoniaca è utilizzata nei prodotti per la pulizia domestica, nella produzione di fertilizzanti, e come reagente in vari processi chimici dove un’alta basicità potrebbe essere dannosa.
Applicazioni dell’Ammoniaca
Produzione di Fertilizzanti
L’ammoniaca è un componente chiave nella produzione di fertilizzanti. È utilizzata per sintetizzare composti come il nitrato di ammonio (NH4NO3) e l’urea (CO(NH2)2), che forniscono azoto essenziale per la crescita delle piante.
Pulizia Domestica
L’ammoniaca è spesso presente nei prodotti per la pulizia grazie alla sua capacità di dissolvere grassi e neutralizzare odori. Le sue proprietà basiche aiutano a scomporre le macchie e i residui organici.
Trattamento delle Acque
L’ammoniaca è utilizzata nel trattamento delle acque per regolare il pH e rimuovere contaminanti come i nitrati. Le sue proprietà basiche la rendono efficace nel neutralizzare acidi presenti nell’acqua.
Industria Chimica
L’ammoniaca è un importante reagente nella sintesi di numerosi composti chimici, tra cui esplosivi, coloranti e plastica. La sua capacità di agire come base debole è utile in reazioni dove una base forte potrebbe causare decomposizione o reazioni indesiderate.
Sicurezza e Maneggiamento dell’Ammoniaca
Nonostante le sue numerose applicazioni, l’ammoniaca può essere pericolosa se non maneggiata correttamente. È essenziale seguire le linee guida di sicurezza per evitare rischi per la salute.
Ventilazione
L’ammoniaca è un gas pungente che può irritare le vie respiratorie. È importante utilizzarla in aree ben ventilate per evitare l’accumulo di gas.
Dispositivi di Protezione Individuale
Quando si lavora con l’ammoniaca, è consigliabile indossare guanti, occhiali protettivi e, se necessario, maschere respiratorie per evitare il contatto diretto con la pelle e gli occhi.
Stoccaggio
L’ammoniaca deve essere conservata in contenitori sigillati e resistenti, lontano da fonti di calore e fiamme. È importante tenerla fuori dalla portata dei bambini e degli animali domestici.
Pronto Soccorso
In caso di esposizione accidentale, è fondamentale sciacquare immediatamente la zona interessata con abbondante acqua e consultare un medico. In caso di inalazione, spostarsi in un’area con aria fresca e consultare immediatamente un medico.
Conclusioni
L’ammoniaca (NH3) è una base debole secondo le teorie di Brønsted-Lowry e Lewis. La sua costante di dissociazione basica (Kb) relativamente bassa e il pH moderato delle sue soluzioni acquose confermano questa classificazione. Sebbene l’ammoniaca non sia una base forte, le sue proprietà la rendono estremamente utile in molte applicazioni industriali e domestiche. Tuttavia, è essenziale maneggiare l’ammoniaca con cautela per evitare rischi per la salute.
Parole Chiave
- NH3
- Ammoniaca
- Base forte
- Base debole
- Teoria di Brønsted-Lowry
- Costante di dissociazione basica
- p