Polarity of HF Molecule
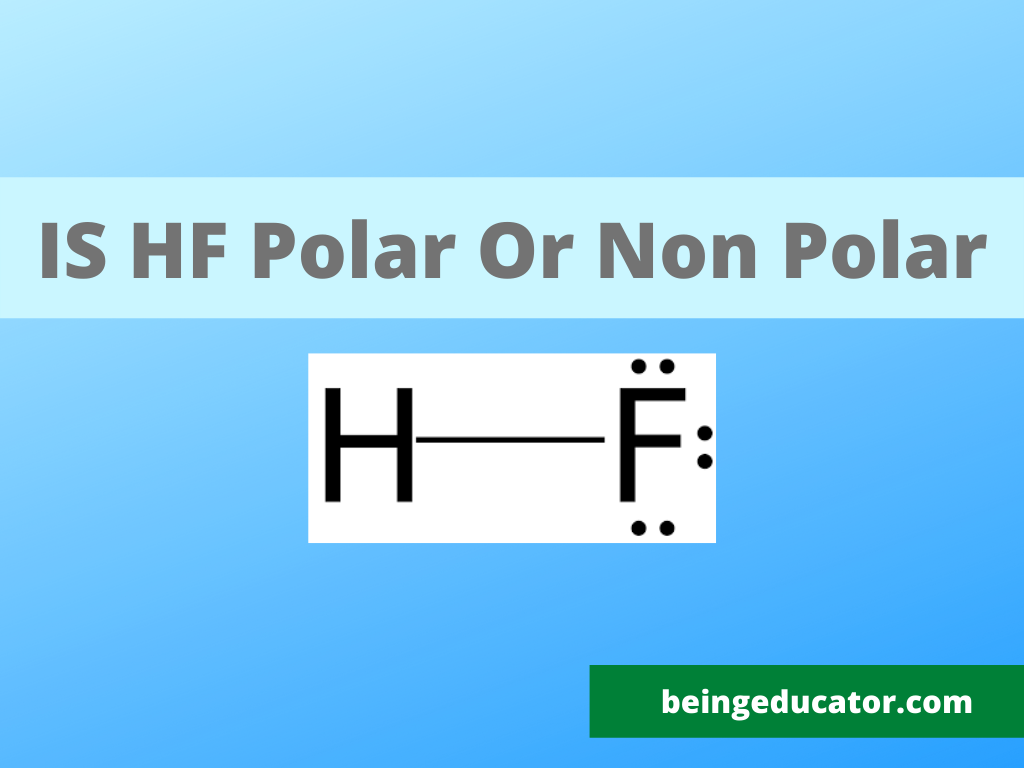
A lot of chemistry graduates ask while solving the lewis structure that Is HF Polar or Non Polar? Hydrogen fluoride is one of the weak acids and is a strongly polar molecule due to the large electronegativity difference between hydrogen and fluorine molecules. The fluorine with the highest electronegative value and hydrogen with a small atomic size being less electronegative element and due to which a large difference in electronegativity value occurs when both hydrogen and fluorine molecules bonded together to form H-F molecule.
Most of the time it is pretty obvious when someone talks about the polarity of the H-F molecule as it is a strong dipole dipole interaction is present among H-F molecules turning it into a polar molecule. The reason behind the polarity is the difference in electronegativity. The same is the case of ammonia molecule as ammonia is a polar molecule due to the high electronegativity difference between hydrogen and nitrogen
Why is HF a polar compound?
HF is a polar molecule because huge electronegativity differences between the hydrogen(2.2) and fluorine(4.0) molecules create two opposing dipoles in the H-F molecule. Fluorine being more electronegative tends to get a partial negative charge and subsequently, hydrogen attains partial positive charges making the entire molecule polar.
What are polar molecules?
The polar compounds are those with dipole-dipole interaction between the bonded atoms or having a polar covalent bond between the adjoining atoms. It is a big misunderstanding that all polar molecules possess the covalent bond because many of the ionic compounds (like sodium chloride/table salt) exhibit polarity among their molecules.
Hydrofluoric acid is prepared when both hydrogen and fluorine molecules combine through a covalent linkage. Hydrogen with only one valence electron shares it with the seven valence electrons of fluorine molecules thus completing the octet and resulting in the formation of the H-F bond.
How to determine a bond polarity in a molecule?
The polarity in the molecule can be determined by the difference in the electronegativity of the bonded atoms. The general rule of the thumb is when the difference exceeds 1.7 the bond is termed as polar.
Why is H-F a polar molecule?
Polarity in a molecule is measured by the actual difference in the electronegativity value of the atoms and in the case of HF the difference is more than 1.7 making it a polar molecule. The logic behind the electronegativity value is that fluorine has 7 electrons in its outermost shell whereas hydrogen has only one electron in the valence shell. The more electronic cloud makes fluorine a partial negative charge when it bonded with the hydrogen and consequently, hydrogen attains partial positive charge making the whole HF molecule into a polar one.
The shape of the H-F molecule
A total of two atoms are involved in the formation of H-F molecules, thus hydrogen fluoride exhibits a linear shape with a bond angle of 180 degrees. The VSEPR theory also suggests the linear conformation or a symmetric geometry in the hydrogen fluoride molecule with tetrahedral shape.
Bond Angle of H-F molecule
The bond angle of hydrofluoric acid 115 and shape is orthorhombic
Is H-F a symmetrical or nonsymmetrical molecule?
The literature and various bond theories like vesper suggest that the H-F molecule is symmetrical in nature and the bond angle is 180 degrees.
What is the difference between hydrofluoric acid and hydrogen fluoride?
Chemically speaking they both are the same and represented by a similar formula but the physical properties differ a lot. Hydrogen fluoride is present in two physical states gas and liquid and when it is mixed in water hydrofluoric acid is formed and is a weak acid.
Summary
HF is a polar molecule and the reason for the dipole-dipole interaction existence is due to the electronegativity difference between fluorine and hydrogen atoms.



3 thoughts on “Is HF Polar or Non Polar”