COCl2 Lewis Structure
Are you looking for a correct and stable molecular structure of the COCl2 compound it is formed by the bonding of three elements viz chlorine carbon and oxygen? The molecular geometry of any compound not only helps to conclude exact information of the structure but the bond-bond interaction. Though many different theories to predict the molecular structure of chemical compounds are given to know more about the shape and nature of bonding lewis’s structure does the perfect job.
The uses of carbonyl compounds in various chemical industries make them necessary to study them. The common name of COCl2 is carbonyl chloride and it is more often used in pharmaceutical industries, for the preparation of herbicides and in the manufacturing of plastics and eye lens industry. You have to follow the proper laboratory standard procedure to handle the COCl2 gas because of its toxic nature.
The molar mass of cocl2 is 92.98g/mole and its trivial name is phosgene. When we talk in detail about the physical properties of COCl2 it is a colourless compound found in the gaseous state of matter. The only weird thing or unpleasant about the phosgene gas is the very throttling smell irrespective of its use in various industrial processes.
How to prepare phosgene gas?
The preparation of cocl2 in both laboratory and industry is mostly done by the chemical reaction of oxide of carbon (Carbon monoxide) with the chlorine gas. The reaction is only possible in the presence of the carbon catalyst.
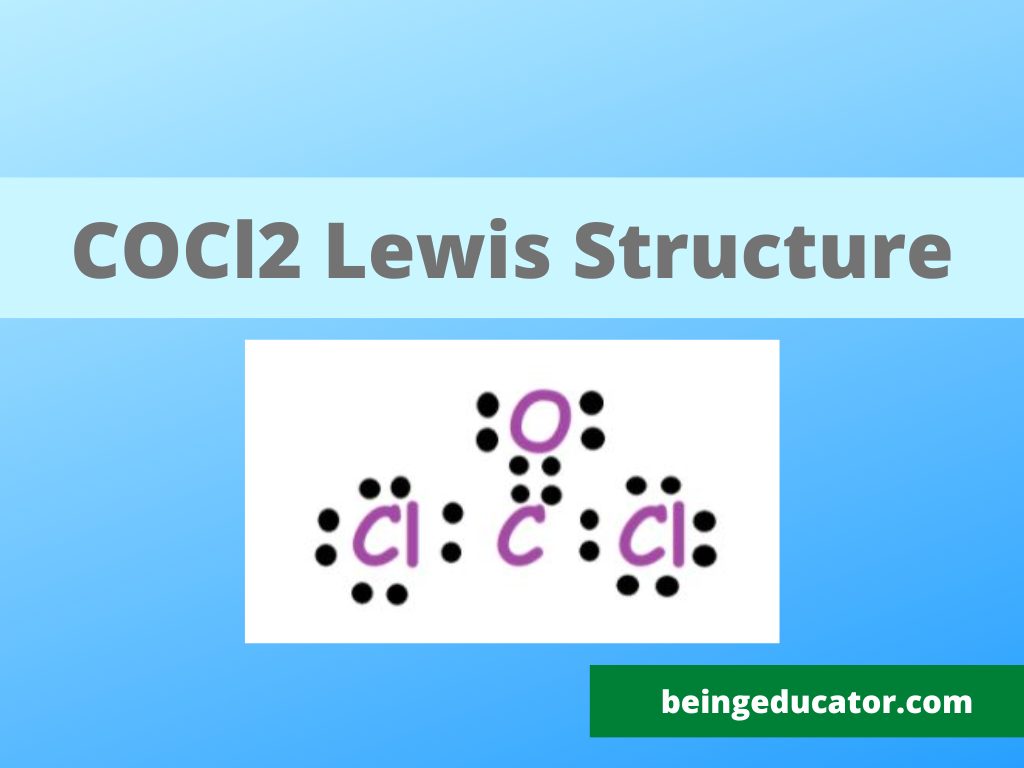
The guide is about knowing more about the molecular geometry and lewis structure of cocl2 gas. If someone asks you what is lewis’s structure you must answer that it is the diagrammatic representation of the structure of any molecule with electron distribution. To draw the correct lewis structure, you must have to know the number of the valence electron in that atom.
Steps of Calculating the Lewis Structure of COCl2.
You need to know more about the valence electrons for finding the correct lewis structure of any molecule and for that reason you can get the help of the periodic table. Carbon with a total of 6 electrons has 4 electrons in the last shell. The oxygen atomic number is 8 and has 6 electrons in the outermost shell. Chlorine belongs to a group of family halogens and they need only one electron to become stable thus chlorine molecule has only 7 valance electrons. The total valence electrons in COCl2 are 24
Now you have to find out which atom comes in the centre of the COCl2 molecule for drawing the lewis structure. For getting to know more about which atom takes the central position we use the electronegativity values and the atom with less electronegative values comes to the centre position and in the case of cocl2 carbon is a comparatively less electronegative element thus it takes the centre position. The other reason we place carbon in the centre of cocl2 is due to the fact it gives stability to the molecule in comparison with other options.
The third step is to calculate the total number of bond pairs in the COCl2 molecule. As carbon is the central atom it forms two bonds with sounding chlorine and a double bond with oxygen. Now octets of all atoms in the dichloromethanal are satisfied.
Now we have to correctly draw the electron in dots for the allocation of bonding electrons. The electron dot diagram shows that the stable version of COCl2 is one with all atoms having 8 valence electrons.
The formal charge distribution is our next step to finalize the lewis structure of COCl2 and the data reveals that the phosgene molecule is neutral and doesn’t have any formal charge on it.
COCl2 Molecular Geometry
Molecular geometry is the 3D representation of the shape of a molecule. The best way to calculate the molecular geometry is by formula AX with the help of VSEPR Theory. The COCl2 molecyke with carbon bein central atom surround with three atoms. There is no lone pair in COCl2 so according to molecular geometry chart it shows A3X where
A= Central Atom
X=No of bond pairs formed by central atoms
E= Lone pair of electron
The COCl2 molecule shows trigonal planer geometry
COCl2 Polarity
A molecule is said to be polar if the electronegativity difference between the atoms is more than 0.5. The value of electronegativity is taken from Pauling Chart. Now there are there types of atoms Carbon (2.55), Oxygen (3.44) and Chlorine (3.16). The electronegativity difference between the Carbon-Chlorine bond and the Carbon-Oxygen bond is more than 0.5 so the molecule is polar in nature. The Molecular geometry is also proof of the polarity of COCl2 because of the asymmetric shape both dipoles are not cancelled this COCl2 shows polarity.
COCl2 Hybridization
The general rule of thumb to knowing the hybridization of a compound is simply knowing the strict number. A steric number is the numerical sum of no of bond pair formed by the central atom and the lone pair of electrons. The steric number of COCl2 is 3+0=3 and it shows sp2 hybridization.
Conclusions
COCl2 scientifically known as dichloromethanal is one with sp2 hybridization that shows polar nature and its common name is phosgene. The lewis structure can be drawn with the help of electronegativity value and valence electron.
Leave a Reply