SiH4 Lewis Structure
Silane is a very unstable and flammable compound used as a doping agent and must be handled with great care. Many students during the chemistry lesson ask me about the SiH4 lewis structure, and I ask them just correctly write the valence electrons of both silicon and hydrogen. You are good to go for writing the exact lewis structure of silane or silicon tetrahalide. If you are looking for the lewis structure of an essential halide of silicon molecule that shows flammable properties, the SiH4 is a significant doping agent. We will discuss the properties of silicon tetrahalide, the lewis structure, molecular geometry, hybridization, and polarity.
A lot of chemistry students not only have limited knowledge of SiH4 but they lack the chemical reactivity knowledge also. Silane when reacting with water it forms a very reactive compound called silanol.
The physical properties of SiH4 indicate that it is a colorless gas with a characteristic smell that resembles the acetic acid and is a compound showing pyrophoric properties. The SiH4 compound, when it comes in contact with the air, it is burst into flames within 5 minutes of its exposure in the air. If you are interested in learning about silane’s manufacturing process, the best way is by the reaction of hydrochloric acid is magnesium silicide.
The molecular geometry of SiH4 is very familiar with the methane as both the compounds show tetrahedral geometry. It is a compound of great industrial importance, but dealing requires excellent care because it may be fatal due to unstable conditions, and this chemical reports several incidents. If you want to know in detail about hydrogen fluoride molecule, I have a detailed topic on Is HF Polar or Non Polar
Steps of writing lewis structure of SiH4
Lewis structure is a diagrammatic representation of compound, atom, or molecule and tells in detail about the chemical pattern of the bonding molecules. You can draw the lewis structure of any molecule perfectly if you are very confident of knowing the valence electrons. Lewis structure tells more about the shape of molecules, hybridization, polarity, and chemical bonding tendencies.
Not down the number of valence electrons in both hydrogen and silicon elements
Identify the central atom for correct bond sharing, molecular geometry, and bond angles.
Now assign the valence electrons in bonding and mark the lone pair and bond pair of electrons.
Check for the charge on atoms, and if there are any charges, then quickly write charge on them.
Look for the stable version of the SiH4 molecule and if the best and suitable stable version of the molecule help in knowing the accurate lewis structure of SiH4.
Step 1
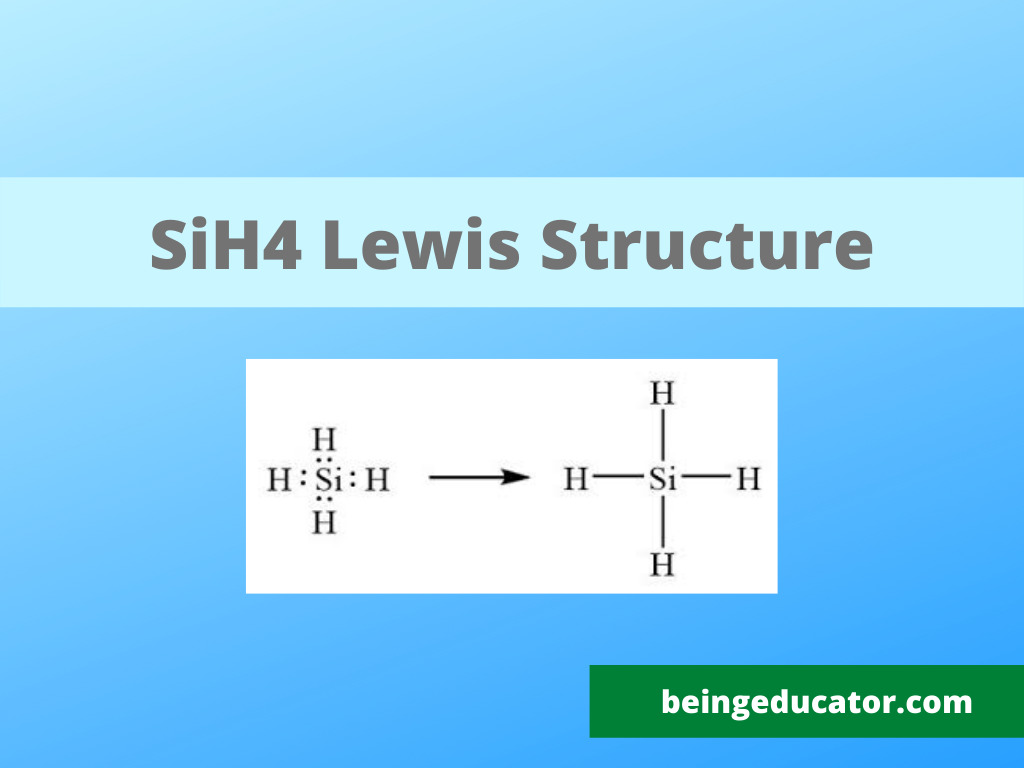
The first and initial thing to remember while writing a lewis structure is to know about the number of valence electrons of all the atoms that take part in chemical bonding. The periodic table tells about the atomic mass and helps in finding the number of valence electrons in the elements. When you write the lewis structure of any molecule, including SiH4, you need to know about the electrons in the outermost shells of all two atoms Silicon and Hydrogen. Silicon has only four valence electrons, and hydrogen has one, so total valence electrons in SiH4 are (4+1×4=8)
Step 2
The central atom in any molecule is mostly the least electronegative element, and in the case of SiH4, the silicon comes to the centre position with the four hydrogen atoms lying around the silicon atoms,
Step 3
The exciting step is writing the lewis structure of any molecule to complete the octet rule. The octet rule is to attain 8 electrons in valence shells to resemble an electronic configuration of the nearest noble gas. When you talk about SiH4, the hydrogen atom needs only one electron to fulfill the octet. In contrast, the silicon atom has four electrons in the outermost shells, so it needs 4 electrons to become stable.
Both hydrogen and silicon share one electron each; an octet of both is completed, with hydrogen having two and silicon having eight valence electrons
Step 4
The formal charge on the SiH4 molecule is zero, so there is no net charge on both the bonding electrons. The general rule of thumb is that molecules with the electronegativity differences showed positive and negative control, but there is no considerable difference of electronegativity; hence there is no formal charge on silicon tetrahalide
Step5
The most stable version of silane is the four hydrogens sounding the silicon at 109 bond angle, and the molecule shape is drawn perfectly to write the correct lewis structure.
Hybridization
The hybridization of sih4 is sp3 and three p orbitals of silicon bonds with the 1 s of hydrogen atoms. The Atomic Oribitols mixing gives the idea of how the atom reacts with others, and also the correct shape of the molecule can be drawn. In chemistry, when we talk about atomic orbital, there are the four names s,p,d,f orbitals where the probability of finding electrons is maximum. Mixing these orbitals may give rise to the sigma bond or pie bond. A sigma bond is the stronger bond with the head on overlapping, while a pie bond is relatively weak and is a parallel bond.
Finding out the hybridization of any halide is much simpler because hydrogen has only one electron is in the s orbital. The silicon atom has 14 electrons, but we talk about the valence electron. There are four electrons. In the ground, state silicon must show 2s2p Atomic orbital, but e has 1s 3p orbital geometry in the excited state. When hydrogen and silicon bond, the sp3 bond is formed, and the silane molecule shows sp3 hybridization.
Molecular Geometry
The shape of the silane molecule is very much familiar with the methane gas and it shows the Tetrahedral Geometry. The VESPER theory confirms the tetrahedral shape of silane molecule and the table is presented below
Polarity
The polar or nonpolar nature of any molecule is directly linked with the electronegative value of these atoms. In usual terms, we learned that the charge on any compound is directly connected with the difference in electronegative values, and there is not a very high difference in electronegative values. The atoms of SiH4 is nonpolar but with slightly positive and negative charges on the hydrogen and silicon atoms, respectively
Summary
silane or SiH4 is a molecule with a tetrahedral shape and is very unstable with flammable properties. The lewis structure diagram shows four hydrogen atoms surrounding the silicon atom. The four valence electrons in the silicon complete their octet by sharing electrons with the four hydrogen atoms.
Leave a Reply