The chemical formula of one of the important organic compounds formaldehyde is ch20 and belongs to the family of organic compounds called the aldehyde. In the laboratory when we remove simple hydrogen from the alcohols aldehyde and ketones are formed. Ch20 has a characteristic pungent smell and has been widely used to preserve food for a longer duration due to its ability to stop the growth of bacteria thus making food eatable for a longer duration.
Formaldehyde is the first member of the aldehyde group and in appearance, it is a colourless gas. Formaldehyde has been used as a treatment against various bacterial outbreaks due to its properties like curing cells and tissues. I am going to discuss in detail the lewis structure, molecular geometry, shape of molecule and polarity of ch2o in detail with step-by-step guidance.
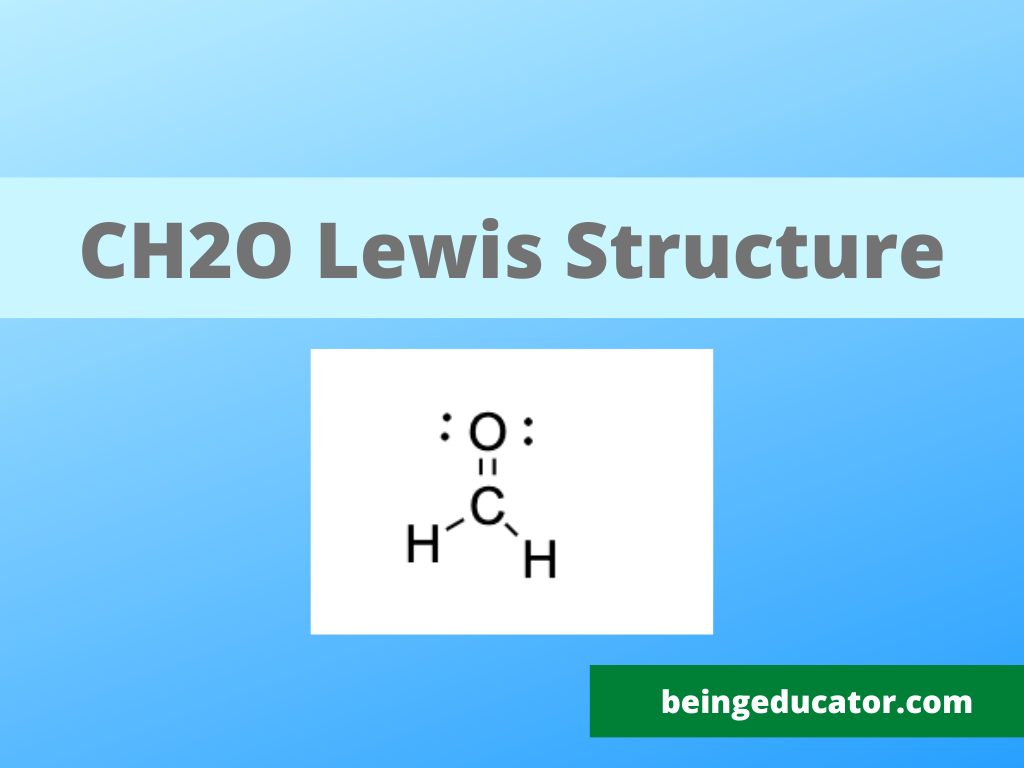
The lewis structure of ch2o not only helps reshape the overall molecular structure but also helps to understand the chemical bonding of the compound. If you are learning to draw the correct lewis structure of any molecule just like ammonia, oxygen or other sulfur oxides, you must know more precisely about the valence electrons.
What is Lewis Structure?
Lewis Structure is the diagrammatic representation of molecular shape and how the valence electrons of all the atoms in a molecule are distributed. Not all atoms behave similarly so you have to be extra cautious to determine the electron geometry, molecular shape, polarity of molecule and chemical properties with the help of lewis structure.
How do atoms make chemical bonds?
Chemical bonds are formed when two atoms have tendencies to share their valence electrons to form a stable molecule. In general, bonding is formed when two atoms share, lose or gain electrons to form a stable ion and satisfy their octet.
What are the valence electrons?
Valence Electrons are the electrons present in the last shell of an atom and these are the electrons that actually help their respective atoms to form a chemical bond and thus move towards stability.
What is the octet rule?
The octet rule is the completion of the outermost shell with a total of eight electrons(exceptions are hydrogen and helium) of atoms or molecules so that they are chemically stable just like the noble gases.
Steps Of Drawing CH2O Lewis Structure
- Estimate the number of valence electrons of hydrogen, oxygen and carbon.
- Locate the central atom for the correct structure of the molecule
- Assign bond pair and lone pair of electrons
- Now easily assign any charge on the atom for the correct lewis structure
- Check for the stable version of the molecule.
Let’s discuss in detail the lewis structure of CH2O
Step 1
When we draw the lewis structure of ch20 we have to find out the number of valence electrons in all the four bonding atoms. Hydrogen has 1 valence electron in each oxygen has 6 and carbon has four then total valence electrons in ch20 are 12 (2×1+4+6=12)
The eight electrons are needed to meet the criteria of the octet rule and four electrons are surplus that does not take part in chemical bonding. It is worth mentioning that carbon and oxygen are bonded by a double bond
Step 2
The atom with the least electronegativity value comes in the centre and in the case of ch2o carbon has a minimum electronegative value and comes in the centre.
Step 3
Formaldehyde also called methanal composed of two hydrogens and one molecule of carbon and oxygen. Carbon needs 4 bonds to become a stable one thus the tetramolcule methanal has only three atoms. The carbon and oxygen form a double bond thus octet of both are satisfied. A single bond is present between carbon and two hydrogen atoms.
Step4
The formal charge calculation not only helps the molecule to calculate the net dipole moment but many physical and chemical properties of the atoms are
Step 5
The last step is to check for the most stable version of the methanal. The stable lewis structure for the formaldehyde molecule is one with
Hybridization
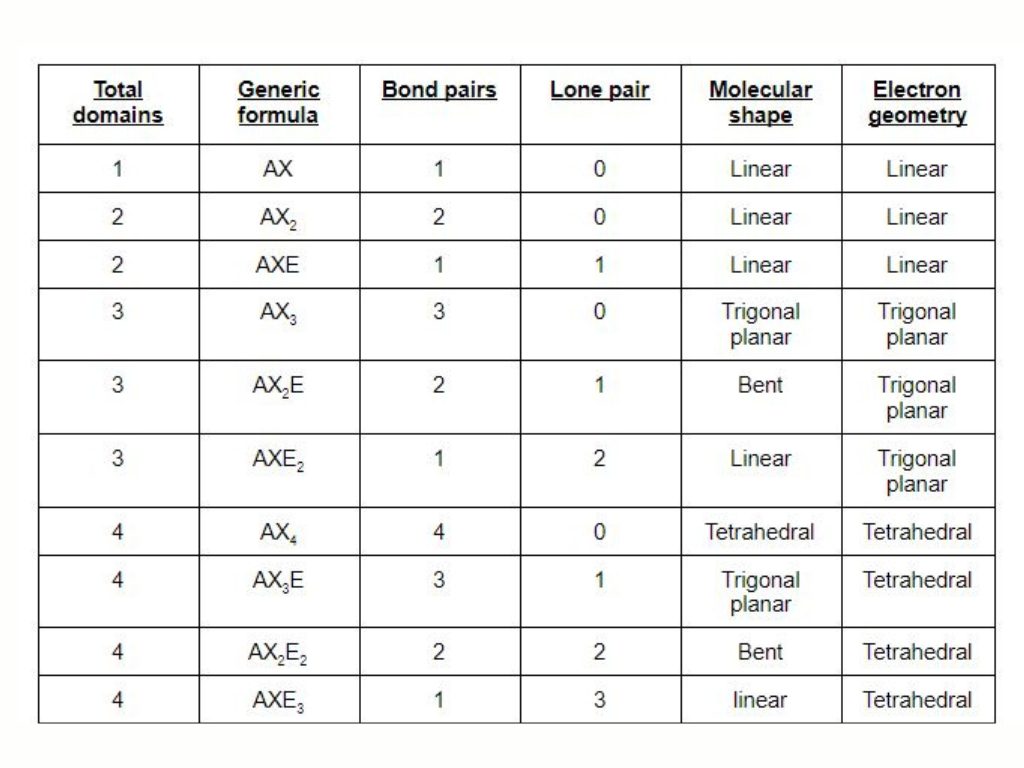
The hybridization can be calculated by simply counting the steric number. The steric number is the sthe um of sigma bond and lone pairs of electrons present on the central atom. Carbon has three sigma bond one with oxygen and two with both hydrogen atoms with no lone pair thhus the steric number is 3+0=3. The molecule of ch20 shows sp2 hybridization
Geometry of Molecule
The molecule of formaldehyde is one of the tetramolecule(4 atoms) and has two types of bonds H-C-H and H-C-O bond angles of bond H-C-O and H-C-C are 122 and 116 respectively. It is very simple to know such deviation of the bond angle and the reason is the lone pair of electrons on the oxygen molecule that make a huge shift in the change of molecular structure
The major way of huge sift in 120 bond angle is the availability of lone pair of electrons on oxygen atom and repulsion causes the geometry to the bent structure.
Polarity of Molecule
CH2O is a polar compound and shows a dipole moment. Polarity is the existence of dipole moment on the bonding atoms and the major reason for this is the difference in electronegativity values in both atoms. The oxygen atom is more electronegative than both carbon and hydrogen and there are two dipoles present in the formaldehyde molecule. If you want to learn about the polar nature of hydrogen fluoride I have published a blog post. You can visit the page Is HF Polar or Non Polar for a detailed explanation.
Leave a Reply