SO3 is an oxide of sulfur and is the primary pollutant present in the atmosphere. When mixed with water vapors in the air, Sulfur trioxide leads to the formation of sulfuric acid, thus contributing to acid rain formation. A lot of students ask about the polarity of the so3 molecule. The SO3 molecule shows nonpolar nature as the electron sharing among the sulfur and oxygen molecules is equal, and thus no dipoles are created. If a student asks about the examples of polar compounds, the very first name came to our mind is PCl3

The SO3 due to its trigonal planar shape is a nonpolar bond each S-O atom bonded with each other at a directed 120° canceling the singular polarity of S-O electronegativity difference. Hence SO3 is considered as a nonpolar linkage
The confusion among the students is pretty obvious regarding the polarity of the so3 molecule. This confusion is due to the difference in electronegativity between oxygen and sulfur molecules. The electronegativity difference may cause any molecule to be polar. Still, in this case, the three S-O bonds lie at 120° making the shape trigonal planar, and thus the sulfur trioxide molecule is nonpolar.
The geometry of the so3 molecule is like an asymmetrical structure with the sulfur atom bonded with three oxygen atoms to give a trigonal planar arrangement. The lewis structure of so3 explained the shape with sulfur atoms surrounded by three oxygen atoms.
If we look at the electronegativity difference between sulfur and oxygen the so3 molecule must be a dipole but in general, it is not a dipole due to its shape as all the three S-O bonds cancel each other polarity
Sulfur trioxide exists in all three physical states like solid, liquid, and gas. In a solid-state, it occurs in the form of crystals, but in the majority, it is found in nature in gaseous form. Though it is regarded as one of the major air pollutants, the primary source is the exhaust of automobiles and chimneys of the factories.
The shape of sulfur trioxide is trigonal planar with an S-O bond angle of 120° each
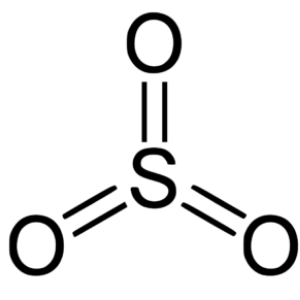
The oxygen and sulfur both have 6 valence electrons, and each oxygen atom has two lone pairs of electrons that turn into a regular asymmetric molecule. The usual S-O atom is polar in nature but each bond cancels the polarity of each bond making SO3 a nonpolar entity.
Another explanation of the nonpolar behavior of so3 is the equal sharing of electrons between the sulfur and oxygen atoms. In simple terms, the molecule is termed as polar when there is unequal sharing of electrons between both bonded atoms but in this case, each atom has equal electron sharing thus the so3 molecule is considered as a polar molecule.
Why is SO3 nonpolar?
In Fact, the large electronegativity difference between Sulfur and oxygen makes a single S-O bond polar but the three S-O bonds with a bond angle of 120° cancel the individual’s polarity and hence make the sulfur trioxide a nonpolar molecule. So the confusion regarding SO3 polar or nonpolar is cleared by this explanation.
Chemical Formula of Sulfur trioxide
Sulfur trioxide is chemically written as SO3 and is commonly known as sulfuric oxide or sulfuric anhydride.
Geometry of SO3
The shape of the molecule explains the nature, as depicted by the trigonal planar shape with a 120° S-O bond angle. The shape of SO3 atoms is asymmetric, and thus the net electron cloud distribution is zero.
SO3 molecular shape
The three oxygen and sulfur bonds in a sulfur trioxide give them a trigonal planar shape, and the pure symmetry of these molecules makes it a nonpolar compound. Usually, the significant differences in electronegativity yield polarity in any molecule, but in this case, the molecule’s shape explicitly explained the nonpolar behavior of SO3.
Summary
Students most of the time confuses about whether so3 is polar or nonpolar due to the high S-O electronegative difference. Sulfur trioxide is one of the major air pollutants and the main source of it is the burning of fuel in an automobile engine. When mixed with water vapors in the air it forms sulfuric acid and leads to the formation of acid rain. It is a nonpolar molecule because the shape of the molecule cancels the high polar S-O bonds and makes it a nonpolar molecule despite the large electronegativity difference between oxygen and sulfur molecules.
Leave a Reply