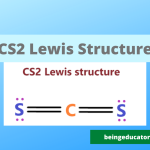
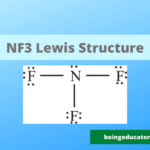
Lewis Strucutre of I3 Molecule
The molecule of i3, formally called triiodide, comprises three iodine atoms with a net negative charge. The only reason to study this chemical entity is due to the fact it is widely used in many identification reactions of different atoms. Many important salts also contain the triiodide molecule due to its characteristics formation of ionic compounds. The molar mass of the triiodide ion is 380.7134 grams. In this article, we are discussing in detail the I3 Lewis Structure to clarify the ambiguities of students
The correct triiodide lewis structure is only evident when knowing the number of valence electrons in all bonding atoms. However, most chemistry students are aware that the correct lewis diagram can only be drawn if the first step of knowing the valence electrons of the atoms is done correctly. In this webpage, I am going to explain the lewis structure of triiodide ion.
What is lewis’s structure?
Many chemistry students know the lewis diagram, but only a few know that the lewis diagram can only be drawn if we know proper structure and bonding information. The lewis structure is the graphical representation of atoms of a molecule and with complete sharing of electrons.
Steps of drawing lewis structure of I3
The lewis structure diagram efficiently predicts the chemical reactions and nature of reactivity. To know more about the i3 lewis dot structure, we have to discover the valence electrons of i3. The significance of valence electrons is self-evident because they take part in chemical bonding
· Locate the number of valence electrons in I3
· Identify the central atom in i3
· Now assign the lone and bond pair for the correct diagram
· Now check for the presence of formal charge in i3 molecule
The final step is to find out the stable form of the molecule
Step 1
A number of valence electrons in Triiodide atoms will be correctly known if you remember the valence electrons in the iodine atom. The iodine atom has a tendency to gain one electron to complete its octet because there are seven valence electrons in iodine atoms. Iodine belongs to the family of compounds known as halogens (salt formers). Now the triiodide molecule has three iodine molecules with a negative charge means they have one extra electron. The total number of valence electrons in triiodide molecule is
7×3=21+1=22
What is Octet Rule
Gaining 8 electrons just like the noble gases is known as octet rule and iodine, being part of the halogen family, needs only a single electron to complete its octet. The molecule always shows stability when it gains electrons, loses electrons, or shares electrons to make a new chemical bond and thus become stable. When we talk about stability, noble gases are the family of compounds more stable because they have a complete outermost shell.
Step 2
The next step is the determination of the central atom, but in this case, all the atoms are identical, so the central atom in i3 is the iodine molecule. The molecule with the least electronegativity value comes in the center but all the iodine molecules are in triiodide ion thus this step is way easier than other steps
Step 3
Now it is time to assign the bond pair and lone pair in the I3 molecule for the valence shell. The octet rule states that each atom must have 8 valence electrons but the iodine molecule has only 7. The central atom gives two electrons to the other two iodine molecules in the triiodide ion and it remains with 6 electrons that are not part of bonding. Thus in triiodide, there are two bond pairs and 3 lone pairs of electrons.
Step 4
The net charge on the triiodide molecule is -1 and making it a polyiodide molecule with a negative charge on it.
Step 5
The most stable form of triiodide molecule is that the linear and symmetrical shape of i3 molecule
Hybridization of I3
When you draw the structure of any molecule, it is the 2d structure, but the hybridization of atoms and molecules can reveal the 3d representation of molecular shape. The hybridization of i3 is sp3d means three orbital take part in forming an i3 molecule viz one s, three p, and one d molecular orbital. The 3d structure of any atom reveals not only the correct shape but also physicochemical properties. Check out more about H2S Lewis Structure.
Many students seek to know how to calculate the hybridization of any molecule correctly. The formula is H=0.5(V+M-C+A), where V=No of valence electrons M=Monovalent atoms, C=Charge of Cation, V=Charge of Anion, and H is the Ultimate hybridization value. In the case of i3- ion, there are two monovalent atoms, V=7 C=0 A=1 and H =5; thus, the hybridization of the i3 molecule is sp3d.
Polarity of I3 Molecule
The real challenge is to call triiodide a polar molecule because many factors confuse the molecule among students. In chemistry, a molecule with some dipole moment is considered the polar one. The other criteria to discuss polarity is the determination of electronegativity difference. The molecule of i3 has a net dipole moment, but it is soluble in water. The shape of the molecule is linear and symmetrical, making it a nonpolar bond. Thus it’s debatable to consider the molecule as polar or nonpolar because it has both possibilities.
Molecular Structure and Shape of Molecule
The lewis dot structure of i3 molecules not only tells more about the bonding electrons but also maintains to establish. The lewis structure of i3 reveals that the occurrence of three lone pairs with each pair tends to repel the others, and thus, more likely, the shape is linear and symmetrical.
Summary
The triiodide ion anion of iodine molecule with three iodine molecules bonding each other and the shape is symmetrical and linear. A molecule’s polarity is debatable, but in reality, it is soluble in water (shows polar characters) and has a net negative dipole with a linear molecular shape.