Krypton difluoride is a chemical name of krf2 and one of the initial compounds prepared for the Krypton one of the noble gas. If you look at the chemical combination of the krf2 compound, it is nonpolar with net dipole zero. Though the fluorine atom is one of the most electronegative elements, the symmetry of the krf2 reveals to be polar, and symmetrical geometry results in the formation of a polar bond.
Physical properties of KrF2
The molar mass of KrF2 is 121.795 grams/mole, and it is the first krypton compound formed on the earth. KrF2 is a colorless but thermally unstable compound of Krypton, and it is mainly used as an oxidizing and fluorinating agent.
Shape of KrF2
The shape of krypton difluoride is the liner, and there is equal electron distribution between fluorine and Krypton.
Bond Angle of KrF2
Though the KrF2 is a nonpolar molecule, and its shape is linear. The bond angle of KrF2 is 180, and it is pretty evident from its molecular geometry.
What are polar molecules?
In chemistry, we call the compounds polar when two dipoles are present, which is the electronegativity difference. The elements with more electronegativity gather more electronic charges and have the net negative charge on them. When the electronegativity difference between two atoms in a particular molecule exceeds 1.70, the molecule is termed a polar entity.
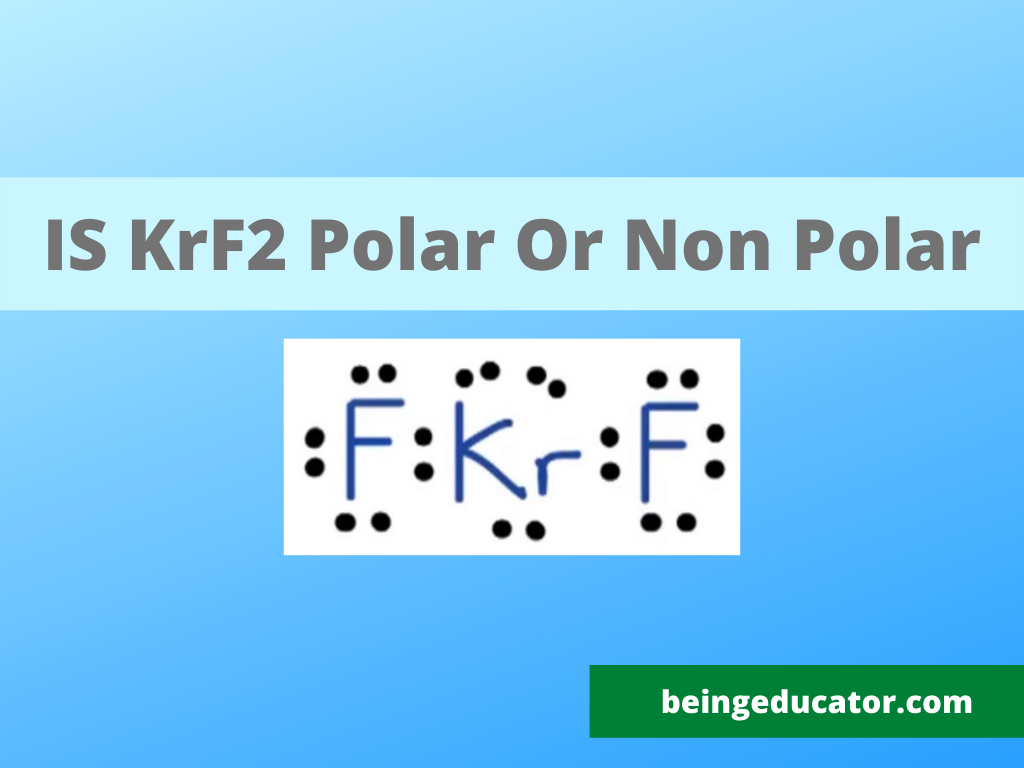
Lewis Structure of KrF2
The KrF2 is attractive due to its nonpolar behavior, though considering the electronegativity difference between Krypton and fluorine, it should be polar, but chemically it is nonpolar and shows linear structure. Its molecular geometry explains the nonpolar behavior of krypton difluoride as it exhibits the liner shape. The two fluorine atoms are bonded with a single krypton atom. Krypton is in the center of two fluorine atoms in KrF2 with equal charge distribution among the fluorine atoms.
The shape of krf2 is linear and is symmetric, with equal charge distribution among the halogen. Make it a nonpolar molecule. We can draw the lewis structure of krf2 by just assigning the lone pair of electrons and valence electrons. The fluorine and Krypton both belong to group 7 and group 8 of the modern periodic table. Kr has eight valence electrons, fluorine has seven valence electrons, and two fluorine molecules bonded with a central krypton atom. Makes a total of 22 valence electrons. After calculation, we have a total of 22 valence electrons in KrF2 (8+14=22).
Now we rearrange the electron distribution as for bonding atoms, only four valence electrons will be used in both fluorine atoms, and 2 electrons in the Krypton will participate in bonding. The remaining 16 valence electrons are not awaiting octet completion. There will be three lone pairs of electrons on the krypton atom, and it can be the best logical explanation of the lewis structure of krf2.
Conclusion
Krypton is one of the noble gases (called rare gases) as they have complete octets in their valence shells. The noble gases have been thought to be nonreactive for decades, and recently some compounds of noble gases were prepared, and KrF2 is one of them. KrF2 shows the nonpolar characteristics and has a liner geometry.



1 thought on “Is KrF2 Polar or Non Polar”