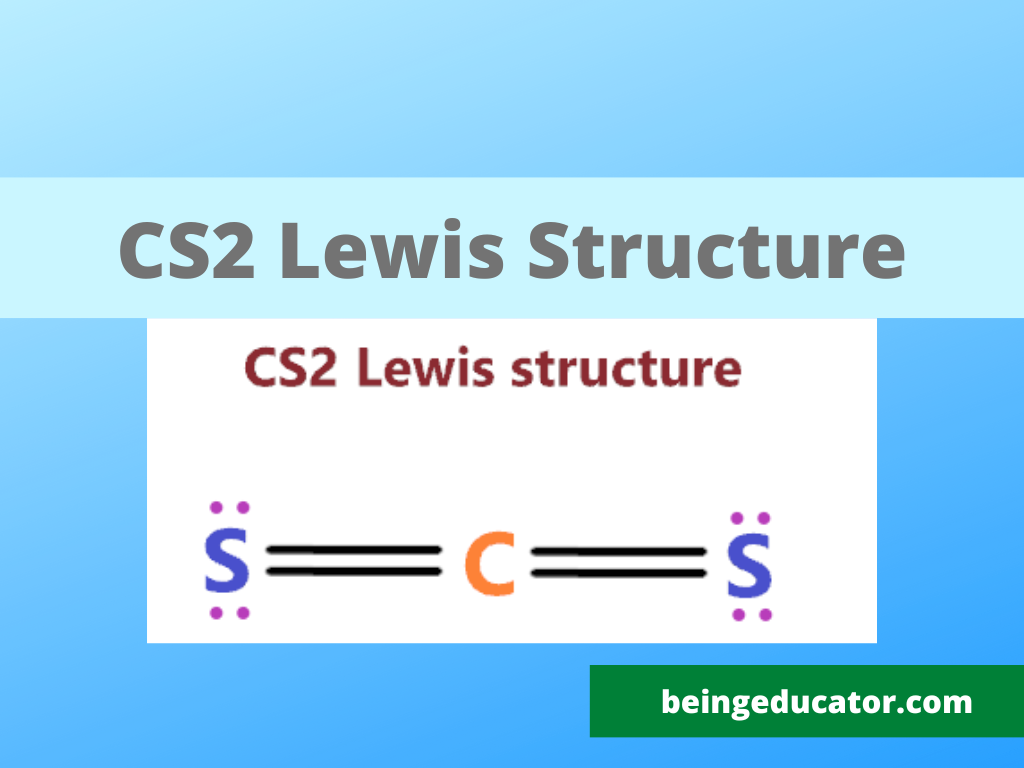
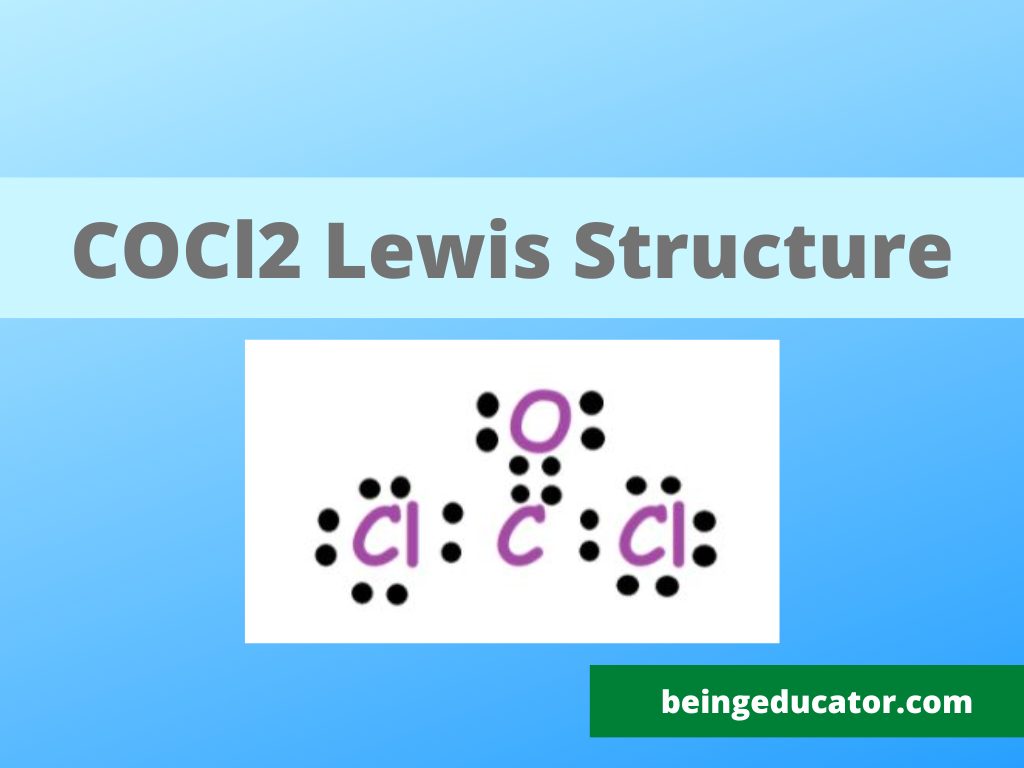
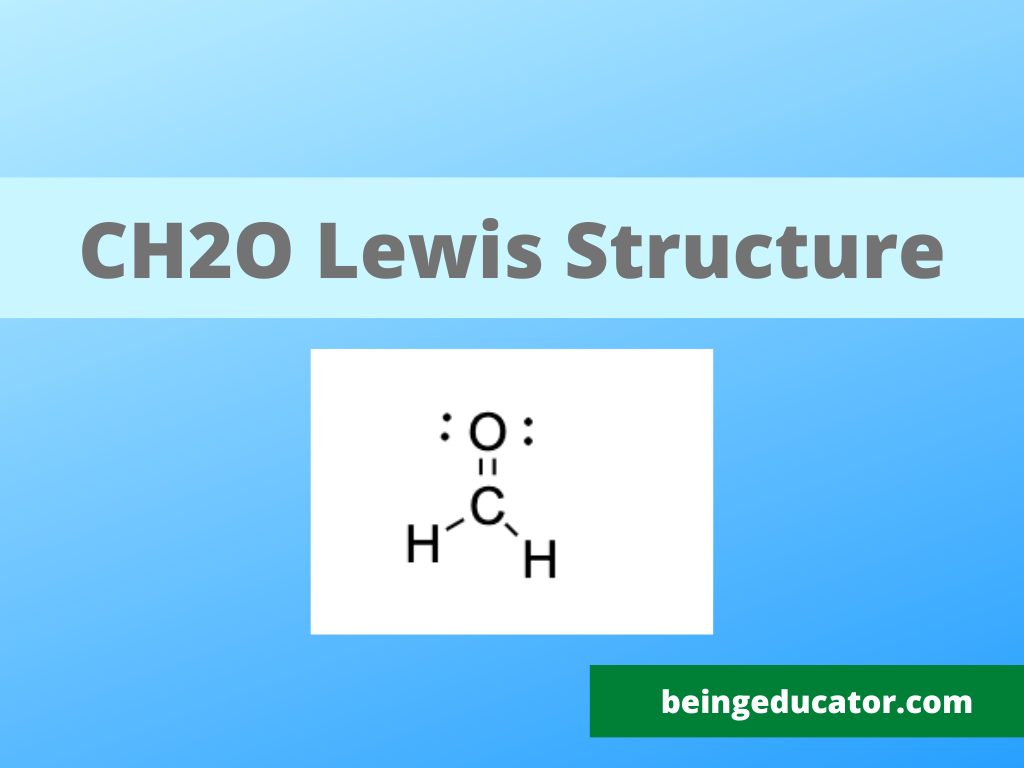
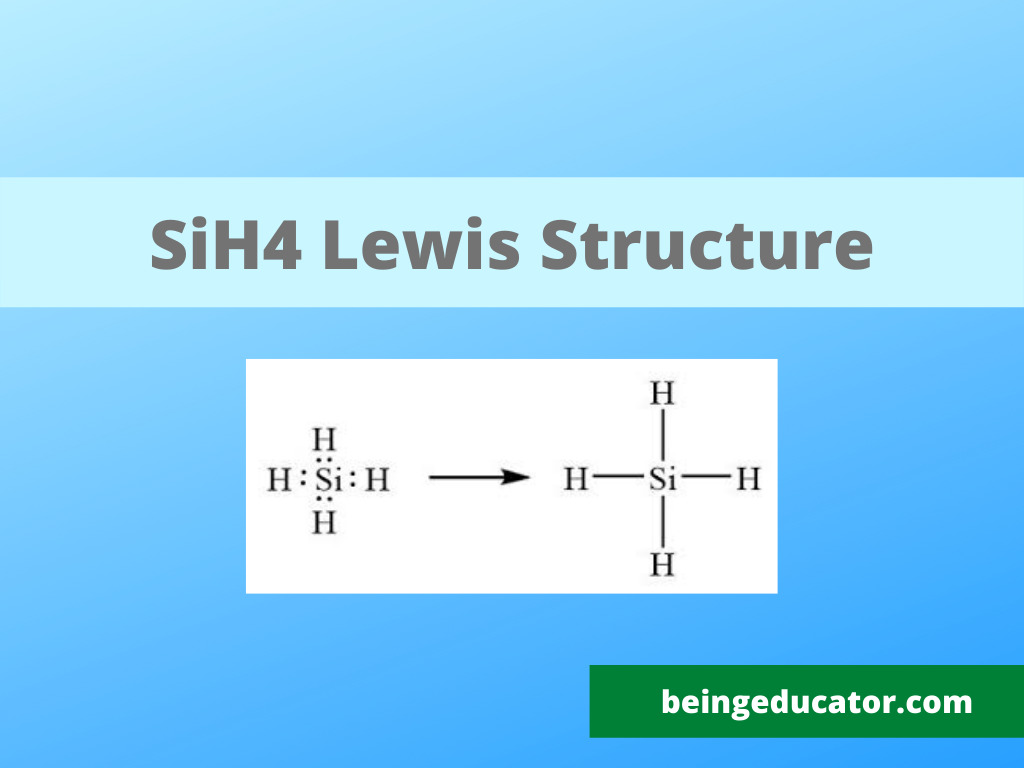
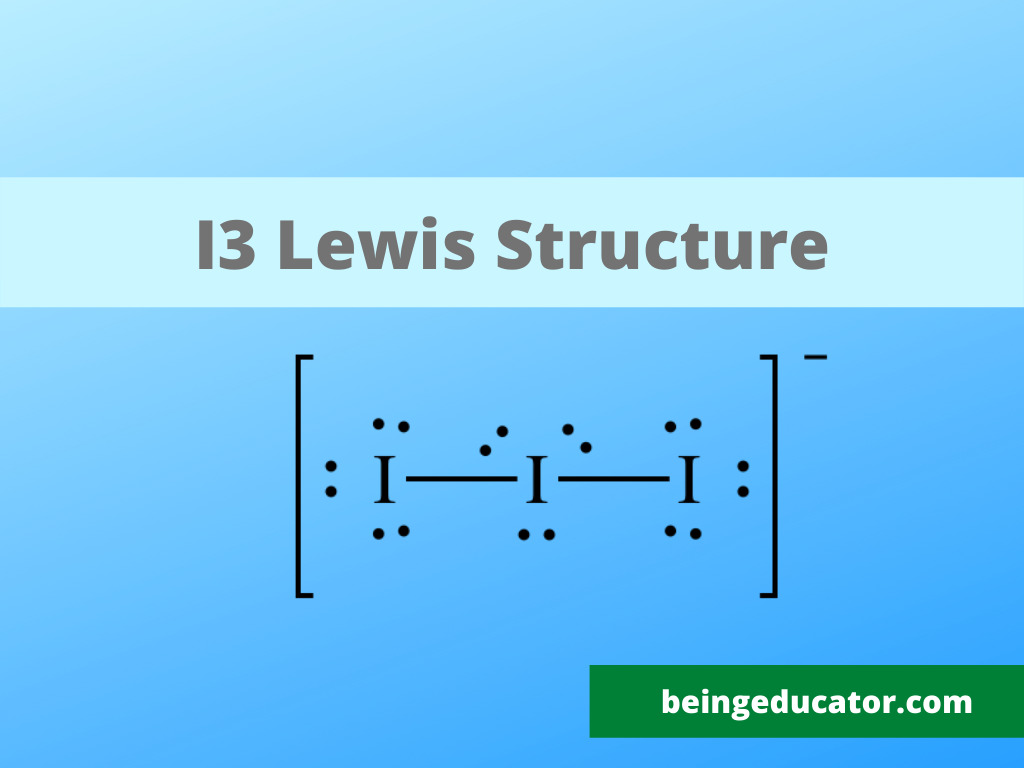
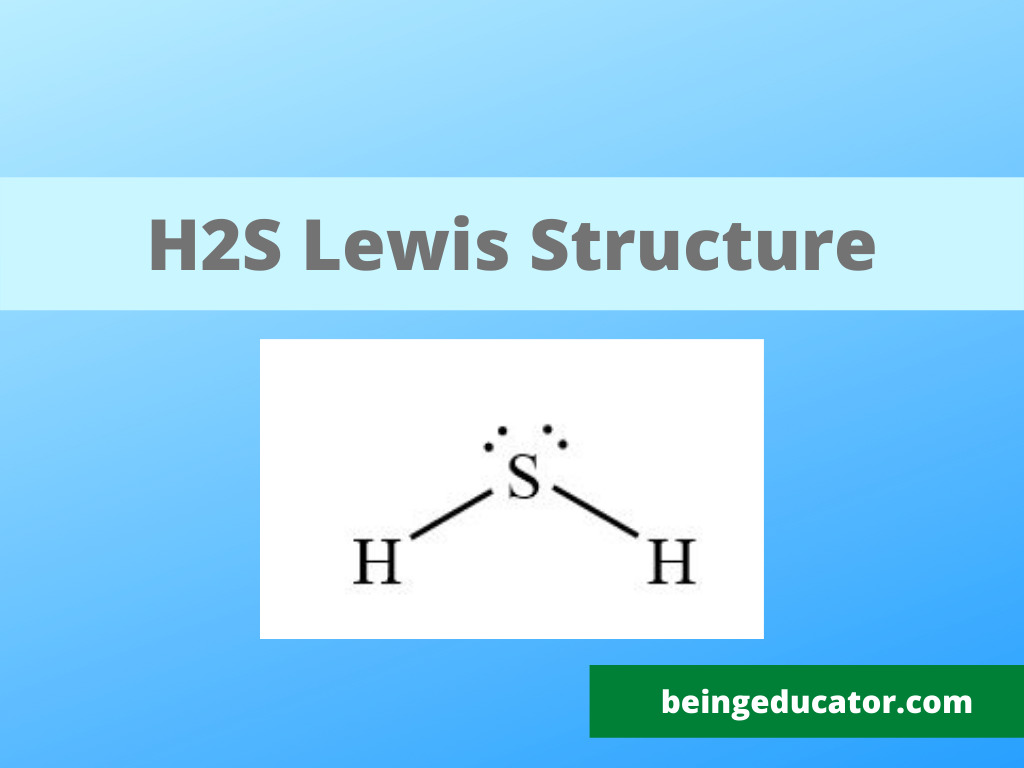
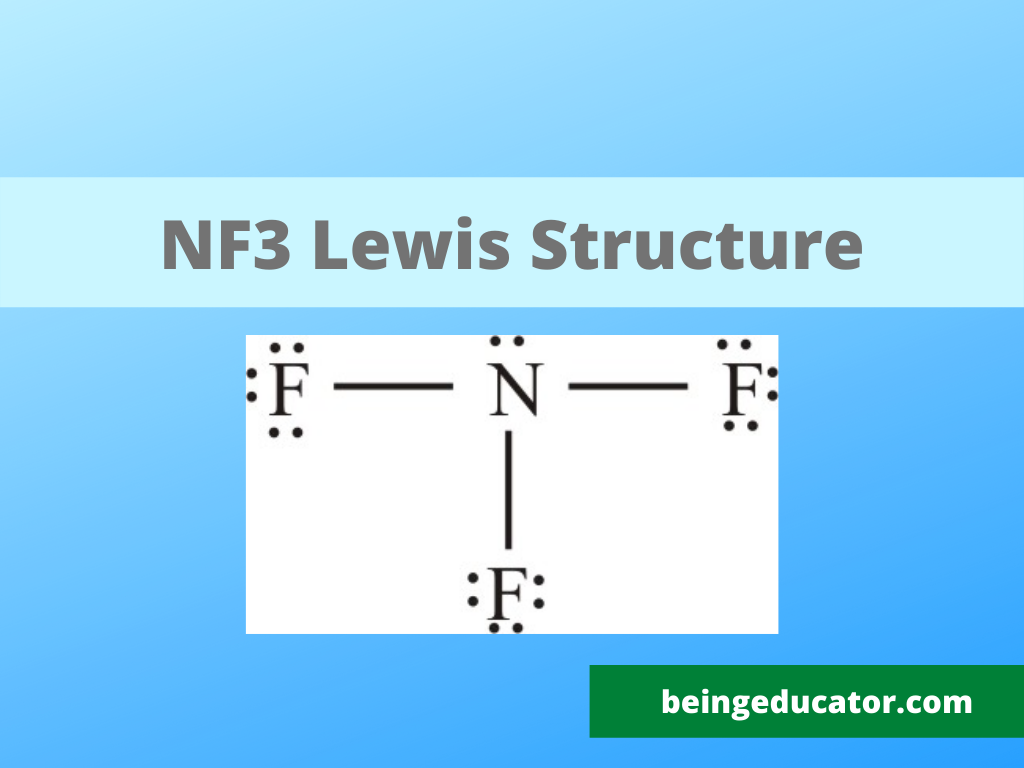
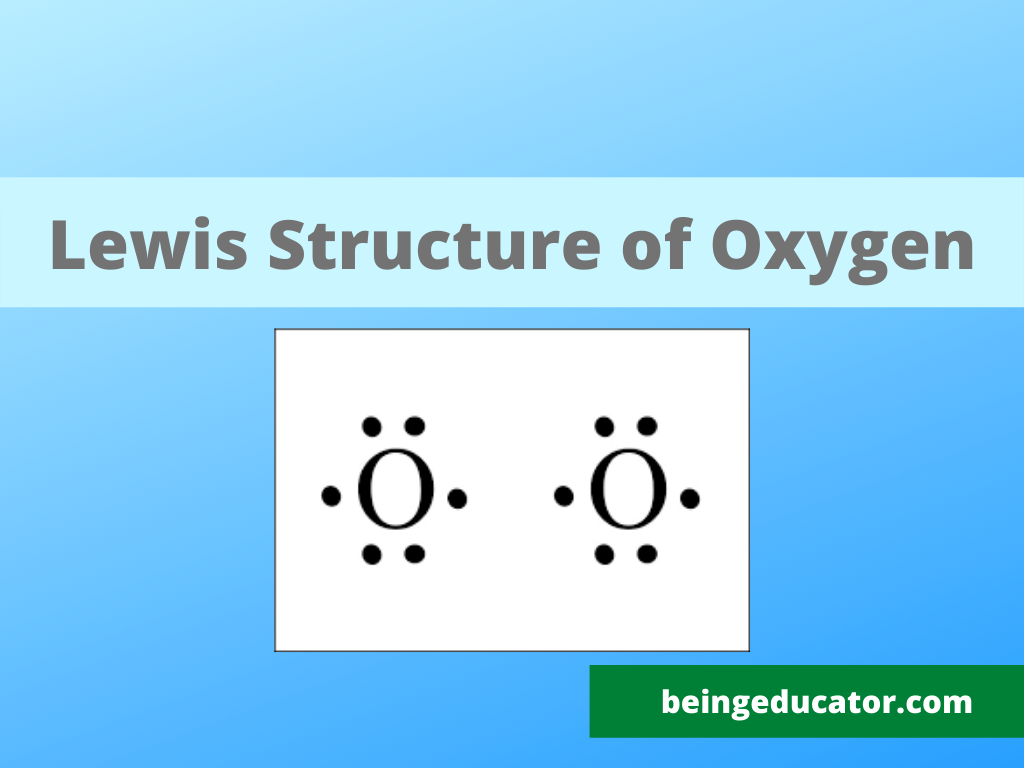
NH3 Base Forte O Debole
L’ammoniaca, con la formula chimica NH3, è una delle sostanze chimiche più comunemente conosciute e utilizzate. Ma la domanda che molti si pongono è: l’ammoniaca è un acido o una base? In questo articolo, esploreremo in dettaglio le proprietà chimiche dell’ammoniaca, la sua natura acida o basica, e le implicazioni pratiche e teoriche di questa … Read more